Atoms are the building block of our universe. Consider the following question proposed by the Greek philosopher Democritus. Imagine if you had the world's sharpest knife. If you were to take any object and cut it in half using that knife, then in half again, and again, and again, would you eventually reach a point in which the object was physically incapable of being cut?"
According to Democritus, the answer to this question was yes. At some point, Democritus argued, the object would reach a point in which it was indivisible. He called that object the atom, which he saw as the smallest possible piece of matter. While we now understand that atoms are not indivisible, for most purposes we can visualize them as extremely small spheres that make up everything in the universe. .
Before we begin explaining what exactly these things are, we have to examine a bit of math. The following equation is known as Coulomb's Law and explains what happens when two charged objects are placed together.
You don't have to worry about what charge is or what the different variables mean at this moment. The important thing to take away from this equation is that objects with the same charge, +/+ and -/-, will repel each other, whereas objects with the opposite charge, +/-, will be attracted to each other.
A proton is a positively charged subatomic (smaller than atomic) particle. Similarly, an electron is the negatively charged equivalent. Finally, a neutron is neutral. All atoms are created by different combinations of these three subatomic particles. Through various experiments, we now know that the structure of an atom to be a tight core of protons and neutrons with a "cloud" of electrons floating around. Since electrons are negatively charged and protons are positively charged, we know from Coulomb's law that they should be attracted to each other.
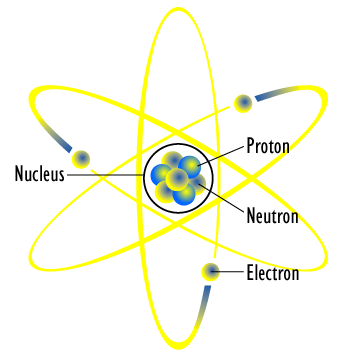
One interesting thing to note is that the radius of the nucleus, where all of the protons and neutrons of the atom reside, is only around 0.0000000000001 meters while containing 99.99% of the atom's mass. If we took that and scaled it up, it would be the equivalent to a pea weighing 250 million tons! While pictures often portray the nucleus as taking up a good portion of the atom's entire size, in reality the nucleus only takes up around 0.001% of the size. This is analogous to having a football field as the atom, with a mosquito at the center as the nucleus; exception in this case, the mosquito weighs more than 100,000 times the entire stadium and everything inside it!
The takeaway from this is that the nucleus, which contains the protons and neutrons, is incredible small compared to the entirety of the atom, but holds the majority of the atom's mass.
another?
Skim through any chemistry textbook or sit in any chemistry lecture and you'll hear or see the word "electron" a couple dozen times and "proton" a handful. Scientists refer to electrons and protons frequently because the properties of matter depend heavily on the behavior of electrons and protons! The differences in behavior of atoms can be attributed in large due to the difference in numbers of protons, electrons, and neutrons from one atom to another. For example, atoms with 7 valence electrons tend to react violently, whereas atoms with 8 valence electrons tend to be gases and not react at all. Using this idea, we can predict whether a specific material will be reactive with something else, if it'll be dangerous for consumption, or any other number of properties just based off the number of valence electrons!
The number of protons that an atom has defines what element it is. The number of neutrons and electrons vary for all elements, but the number of protons stay constant. For example, all atoms with 3 protons are lithium atoms, and all atoms with 8 protons are oxygen. You cannot have an oxygen atom with 3 protons. You can, however, have an oxygen atom with 6 electrons, or 7 electrons, or even 8 electrons. It will still be an oxygen atom because it has 6 protons.

Chances are if you've taken a chemistry class at any point in your life, you've seen the periodic table. The periodic table is an organized table of all the known elements in our universe. The distinction between each of the elements is solely based off the number of protons. As we'll learn later on, the periodic table is the basis of a lot of chemistry as a large number of chemical properties and behavior can be predicted through application of the periodic table.
A lot of what we went over in this post is summarized in an atomic theory proposed by English chemist John Dalton. The theory states the following:
1. Everything is made up of small indivisible and indestructible units called atoms.
2. All atoms of a given element are identical.
3. All compounds are combinations of atoms in specific whole number ratios.
4. Chemical reactions are just rearrangements of atoms.
We've gone over the first 2 parts so far. Part 1 says that atoms make up everything, from the air that we breath to the food that we eat. The 2nd part says that every atom of any element are identical i.e a molecule of nitrogen is the same mass and will have the same properties no matter where the molecule is. Parts 3 & 4 are things that we'll go over in the next section.
1. The 3 subatomic particles are protons, neutrons, and electrons.
2. Atoms are distinguished by the number of protons it has.
3. Dalton's Atomic Theory provides a general summary of how atoms behave.
1. Dalton's Atomic Theory is wrong.
While Dalton's Atomic Theory works for most cases, it is in some ways an outdated theory. We now know that certain postulates of the theory are incorrect. Postulate #1 states the atoms are indivisible and indestructible. With the recent advancements in science, we've been able to identify the materials that make up atoms themselves. Postulate #2 states that all atoms of a given element are identical. We'll soon learn that some atoms of an element can have have a different number of neutrons or electrons, which change the mass and chemical properties. Additionally, postulate #3 is only correct for simple molecules. For complicated molecules such as `C_(12)H_(22)O_(11)`, the ratio is clearly not a whole number.
Dalton's theory provides a backbone of atomic structure that we can later expand on. For this reason, it's important to understand the postulates when first learning about atoms.
2. Why can't you trust atoms?
Because they make up everything!