
If you think back to the first post, we briefly examined a formula known as Coulomb's Law, which stated that things of opposite charge attract while things of the same charge repel. In the case of atoms, we have positively charged protons and negatively charged electrons. These two are attracted to each other.
In the Bohr Model of the atom, protons and neutrons are in the center of the atom while electrons orbit the nucleus at defined distances, similar to how planets orbit the sun. In the picture above, you can see these different orbitals, labelled `n=1,2,3,4...` These orbitals are called shells and the outermost shell is called the valence shell. Electrons inside the valence shell are called valence electrons.
All of the halogens (group 17) are extremely reactive. The reason behind this is that elements of the same group have the same number of valence electrons. F has the same number of valence electrons as Br. Similarly, Be has the same number of valence electrons as Mg, Ca, and Sr. For elements of the same group, the total number of electrons will be different, but the number of valence electrons will be the same. In other words, the total number of electrons doesn't matter (yet). Only the ones in the outermost shell contribute to an element's chemical properties.
Valence electrons have this interesting behavior which is described by the octet rule. The octet rule states the following:
All elements want to have 8 valence electrons.
If you look back at the periodic table, you'll notice that the elements with 8 electrons are in group 18, the noble gases. The octet rule can therefore also be stated as follows:
All elements want to be like noble gases.
This "want" to become like the noble gases is why different elements behave differently. Group 17 elements have 7 valence electrons and strongly desire one more to satisfy the octet rule, going as far as to rip the electron from another neighboring atom. This is why halogens are so reactive: they desperately want one more electron and will often times break apart the surrounding atoms in order to get it. The noble gases are not reactive at all because they have already satisfied the octet rule. It is because of this that they are called noble gases: the noble do not react to the problems of the commons (apparently).
A large portion of an element's behavior can be predicted solely by its number of valence electrons. In fact, there's a measure of how much a specific element wants electrons, called electronegativity. Electronegativity is a measure of how selfish and greedy an element is for electrons. Once an electronegative atom gets hold of an electron, it will not let go. Imagine that there's a really frugal person who's saving up for a new car. This person is likely to hold onto all his money while taking money from whereever he can. On the other hand, imagine a wealthy person who has more money than he needs. He really wants to be charitable and give away some of his money (fictional scenario). The frugal person is the electronegative atom, and the wealthy is the opposite (electropositive).
Electronegativity has been quantified into what is called the Pauling Scale of Electronegativity:
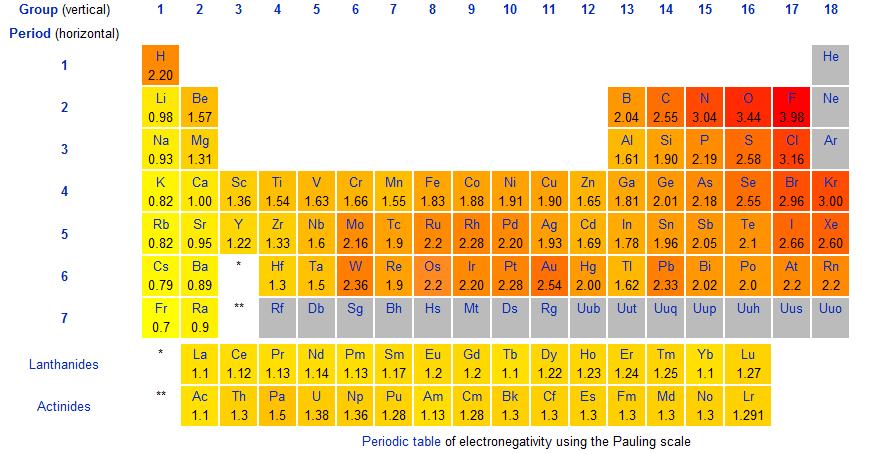
As seen on the modified periodic table, group 17 elements have higher electronegativity values than group 1 elements. The trend for electronegativity is as follows:
F is the most electronegative element, and Fr the least. If you draw an arrow from Fr to F, you will have described the trend for electronegativity.
You can probably see this on the scale itself: the closer an element is to F, the more electronegative it is. Elements with high electronegativity will hold onto their electrons tightly while searching for more electrons. Elements with low electronegativity, also called electropositive elements, will do the opposite by readily freeing up their valence electrons to be taken. This explains why compounds like NaCl exist in such abundance: the Cl atom, being highly electronegative, really wants an electron whereas the Na atom, being low in electronegative character, really wants to give an electron away. Na can then give one electron to Cl to satisfy both their octet rules. Later on, we'll use the concept of electronegativity to explain bonding, or why certain elements pair together.
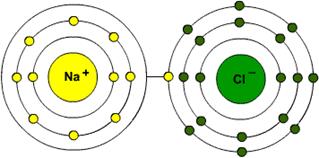
The sodium atom gives away one electron from its valence shell to the chlorine atom, which is missing one valence electron. By doing so, both sodium and chloride satisfy their octets.
Electronegativity is a very important concept in chemistry. To summarize it:
1. Electronegativity is a property of an atom that describes how much it wants electrons.
2. The trend for electronegativity points from Fr, francium, to F, flourine. Fluorine is the most electronegative element while francium is the least.
3. Elements with high electronegativity will want to take electrons from other atoms, whereas elements with low electronegativity will easily give their electrons away.
4. The Pauling Scale of electronegativity quantifies how electronegative each element is. The higher the value, the more electronegative.
1. The Bohr Model of the atom states that electrons orbit around the nucleus in certain distances.
2. The outermost shell of an atom is called the valence shell. The electrons in this shell are called the valence electrons.
3. According to the Octet Rule, all atoms want 8 valence electrons. Elements in the same group behave the same way because they have the same number of valence electrons.
4. Electronegativity is the measure of how selfish and greedy an atom is for electrons. An element with a high electronegativity will hold onto its own electrons tightly and try to take the electrons of other atoms, whereas an element with a low electronegativity will try to give electrons away. The closer the element is to F on the periodic table, the more electronegative it is.
#1. Sodium Chloride
NaCl, also known as sodium chloride, is one of the most widely produced and used compounds today. In fact, chances are you consumed some of it just recently! Sodium chloride, also known as table salt, is used throughout the world as a preservative and as a seasoning. Fun fact: only around 6% of the world's NaCl is used for human consumption! 8% is used for defrosting roads, which we'll explore near the end of the course, and the rest is used for industrial purposes.
#2. Fluorine
Fluorine is in an interesting spot in terms of electronegativity. If you look on the Pauling scale, fluorine has the highest electronegativity of any element, hands down. However, fluorine is such a small molecule that, even if it pulls hard on electrons, the small size means that it cannot hold onto all of the electrons very well. This leads to fluorine having several unique properties.