When an acid dissociates according to the following general equation:
`HA +H_2O ↔ H_3O^+ + A^-`
`A^-` is called the conjugate base of `HA` .
Whenever an acid dissociates, it forms a conjugate base. The acid and conjugate base form a conjugate pair. For example, in the dissociation of `H_ 2CO_3` ,
`H_2CO_3 +H_2O↔ H_3O^+ + HCO_3^-`
`H_2CO_3` is the acid while `HCO_3` is the conjugate base. The two of them form a conjugate pair.
The reason that the deprotonated acid is called the conjugate base is that in the reverse reaction, the deprotonated acid now behaves as a base. `HCO_3^-` is able to accept a proton in the reverse reaction to form `H_2CO_3` , which, according to the Bronsted-Lowry definition, is exactly what a base does!
We can express this in the following "statement":
`"Acid" + "Water" ↔ "Conjugate Base" + "Hydronium Ion"`
Notice that there are actually two conjugate pairs in this reaction: Acid-Conjugate Base, and Water-Hydronium Ion. If you'll notice, the conjugate pairs always vary by one proton. Water (`H_2O`) and Hydronium Ion `H_3O^+` vary by one proton. In this situation, the hydronium ion is the acid and water the conjugate base.
Depending on the strength of the acid, we can determine the strength of the conjugate base. If the acid is strong, the conjugate base will be weak. The dissociation of `HCl` for example:
`HCl + H_2O ↔ H_3O^+ + Cl^-`
The acid-conjugate base pair is clearly `HCl` and `Cl^-` . If the acid is strong, we know that the equilibrium strongly favors the right side. This means that the reverse reaction is not favored and thus, `Cl^-` must be a weak base.
Now consider the dissociation of a weak acid:
`CH_3COOH + H_2O ↔ H_3O^+ + CH_3COO^-`
Since we know that the acid is weak, we know that the reaction favors the left. This means that `CH_3COO^-` is a more potent base, as it reacts with a proton to drive the reaction to the left. We don't say that the conjugate base is a strong base, as that would imply that the reaction is completely to the left. Instead, we say that the conjugate base is stronger in comparison to the acid.
To summarize:
`"Strong acid" + "Water" ↔ "Weak conjugate base" + "Hydronium ion"`
`"Weak acid" + "Water" ↔ "Strong(er) conjugate base" + "Hydronium ion"`
The same concepts apply for bases; just replace acid with base and base with acid in this section. The logic still applies:
`"Strong base" + "Water" ↔ "Weak conjugate acid" + "Hydroxide ion"`
`"Weak base" + "Water" ↔ "Strong(er) conjugate acid" + "Hydroxide ion"`
Here's a table of some common conjugate pairs:
`H_3O^+` |
`H_2O` |
`NH_4^+` |
`NH_3` |
`CH_3COOH` |
`CH_3COO^-` |
`H_3O^+` |
`H_2O` |
`NH_4^+` |
`NH_3` |
`CH_3COOH` |
`CH_3COO^-` |
Neutralization Reactions
If a neutralization reaction reacts to completion (strong acid and strong base in equimolar amounts), the result will be water and a salt. If the neutralization reaction doesn't go to completion, we know that the result can be expressed as:
`"Acid"+"Base" ↔ "Conjugate base" + "Conjugate acid"`
Notice that a salt and water are still technically there, just in the form of conjugate acids and bases.
We can predict which side the reaction favors by comparing the strength of the acids. The position of equilibrium in a neutralization reaction will favor the formation of the weaker acid. Consider the following reaction:
`CH_3COOH + NH_3 ↔ NH_4^+ + CH_3COO^-`
It helps to write out a table:
`CH_3COOH` |
`NH_3` |
`NH_4^+` |
`CH_3COO^-` |
Acid |
Base |
Conjugate acid |
Conjugate base |
`pK_a=5` |
`pK_a=9` |
Remember that the higher the `pK_a` , the weaker the acid. Since the weaker acid, `NH_4^+` , is on the right, the equilibrium position will favor the products.
While the Arrhenius and Bronsted-Lowry models of acids and bases work in most cases, every so often there will be a model that behaves like an acid or base while not conforming to either one of these models. The Lewis Acid-Base Model states that an acid is an electron pair acceptor whereas a acid is an electron pair donor.
In the Lewis Acid-Base Model, acids are electron pair ACCEPTORS while bases are electron pair DONORS
This probably seems a bit counterintuitive as, thus far, all the models of acids and bases involve acids producing something whereas in this model, the acid accepts something. Think of it this way: in the Arrhenius and Bronsted-Lowry models, the acid producers a `H^+` and thereby ends up with a negative charge on the deprotonated atom. In the Lewis model, the acid also ends up with a net negative charge - only instead of this being from losing a positive charge, it comes from accepting a negative charge. The Lewis model can be thought of as an extension to the Bronsted-Lowry model: in the Bronsted-Lowry model, the acid is a positive charge donor while the base is a positive charge acceptor. By donating a positive charge, the acid is concurrently accepting a negative charge. The Bronsted-Lowry and Lewis models are essentially saying the same thing, except that the Lewis model does not restrict the charge transfer to `H^+` .
The power of the Lewis model lies in its versatility. According to both the Arrhenius and Bronsted Lowry models, `BF_3` should not be an acid as it doesn't produce a proton. Experimentally, however, we know that `BF_3` is in fact a weak acid. For example, consider the reaction between `BF_3` and `NH_3` (ignore the bond lengths):
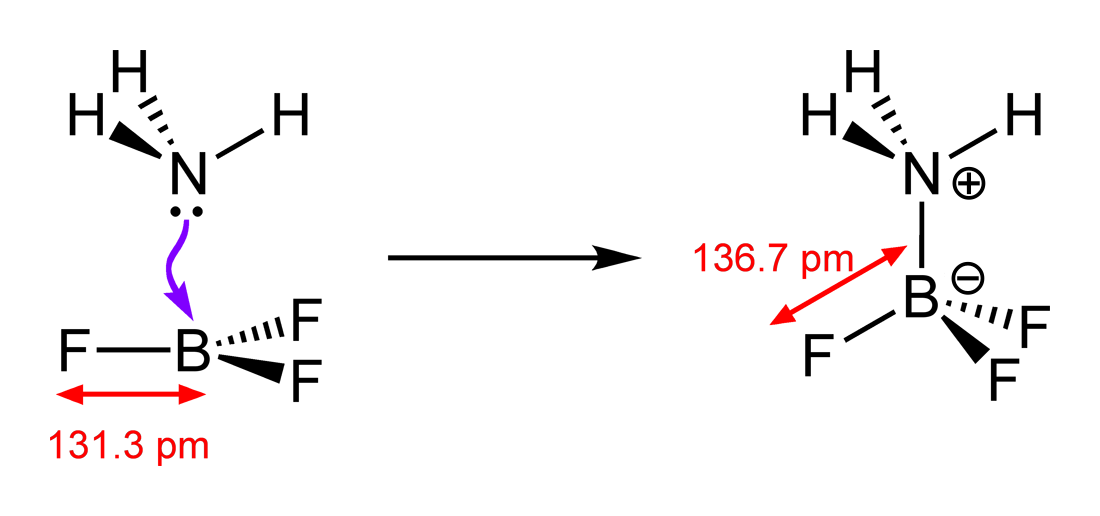
By accepting an electron pair, `BF_3` is able to bond with `NH_3` . The `B` atom incurs a negative charge while the `N` atom incurs a positive charge; just like how normal acid-base reactions end up. This explains why `BF_3` is acidic in solution even without a proton to donate.
Lewis acids are the umbrella under which Bronsted-Lowry and Arrhenius acids and bases fall under. If an acid is a Bronsted-Lowry acid, it must by extension be a Lewis Acid. The significance of the Lewis Acid-Base model lies in its versatility and simplicity. It turns out that many species behave acidically or basically without `H^+` or `OH^-` donations; the Lewis Acid-Base model explains why this is.
To summarize the three models of acids and bases, here's a table:
Model |
Acid Definition |
Base Definition |
Arrhenius |
`H^+` producer |
`OH^-` producer |
Bronsted-Lowry |
`H^+` donor |
`H^+` acceptor |
Lewis |
Electron Pair acceptor |
Electron Pair donor |
1. Whenever an acid dissociates, it forms a conjugate base. Likewise, whenever a base reacts, it forms a conjugate acid.
2. The difference between an acid and its conjugate base or a base and its conjugate acid is one proton.
3. Water and hydronium are a conjugate pair. Hydroxide and water are another.
4. A strong acid will always have a weak conjugate base. A strong base will always have a weak conjugate acid.
5. A weak acid will always have a strong(er) conjugate acid. A weak base will always have a strong(er) conjugate base.
6. The equilibrium position will always favor the formation of the weaker acid or base.
7. The Lewis Acid-Base model explains that acids are electron acceptors whereas bases are electron donors.