Water composes 75% of the world, but not all of it exists as a liquid. Liquid water is the most familiar form since we use it everyday in various forms. In fact, it's so vital to our lives that we as a civilization set up elaborate water transportation networks such that water can be delivered instantaneously to anyone who may need it.
Ice is another form of water which is made by freezing liquid water. The ice comes out harder and colder than the water that initially went in, which indicates some sort of physical change that the water went through.
The last form of water is water vapor, which can be seen when water is boiled. The vapor comes out as a hot gas which makes it painful to come into contact with.
What water, ice, and water vapor all have common is that they are all states of water. When we say state, we're describing the physical arrangement of the material. The most common states of matter are solids, liquids, and gases, though more exist. For now, we'll focus on these three.
Solids have the following properties:
1. Fixed volume and shape
2. Difficult to compress
These properties occur from the microscopic composition of solids. In solids, atoms are packed tightly together with rigid bonds holding them in place. For visualization, here's a microscopic view of table salt, NaCl.

Notice that there's not much distance between the individual atoms. This is because solids are the state of matter where atoms are tightly packed together. This isn't to say that they're packed as tightly as they possibly can be, but that they're close enough and the bonds are rigid enough that they don't move much.
The properties of solids come from this tight packing. Solids retain a fixed volume because the atoms are stable in their arrangement: if you put rock into a mug, the rock doesn't suddenly take on the shape of the mug. The incompressibility of solids follows a similar logic. Since there's little space between the atoms, there is little space for the atoms to compress even when pushed on.
There are two main categories of solids. The first category consists of the crystalline arrangement. For crystalline solids, the arrangement of atoms is uniform all throughout the atom. This means that if you were to take any segment from any part of the molecule, the atoms would be arranged the same way. `SiO_2`, also known as quartz (shown below), is an example of a crystalline solid:

The second category consists of amorphous solids. Amorphous solids are solids that do not have a consistent molecular pattern. In other words, if you were to take a random selection of the amorphous solid from different regions, the samples would not have the same atomic arrangement. `SiO_2`, also known as glass (shown below), is an example of an amorphous solid:
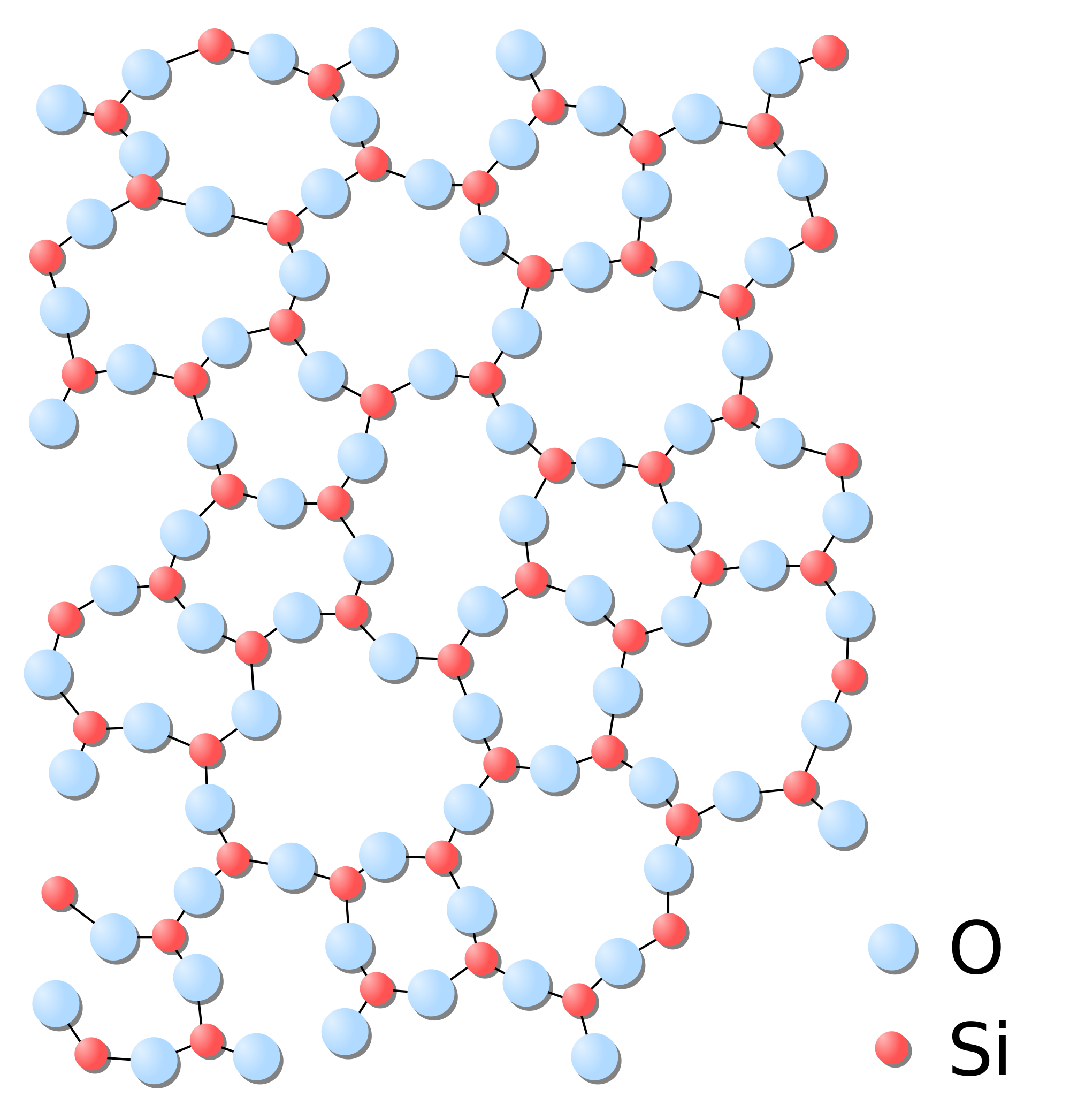
Quartz and glass both have the same chemical formula `SiO_2` but have different physical properties. Glass is used for windows due to its interactions with light that quartz lacks. Quartz is a piezoelectric material which means that an electric potential is created in response to mechanical stress. This ability is why quartz is used as an oscillator, most commonly seen in quartz watches. These differences exist solely due to the difference between the solid being crystalline or amorphous!
Liquids have the following properties:
1. Fixed volume
2. Conform to shape of container
3. Able to flow
4. Difficult to compress
You probably know these just by dealing with liquids on a daily basis. When you pour water into a glass, the water takes on the shape of the glass. When you spill water, it flows and spreads across the surface that it's spilled on. If you were to try to compress water, the water would push back and retain its volume. Notice that the fixed volume and low compressibility are related: if you can't compress something very much, you can't change the volume to any significant extent.

At the microscopic level, atoms in the liquid level are close to each other, but not as close as the atoms are in the solid state(generally). Additionally, atoms in the liquid state are not fixed in position. This is why liquids flow: the atoms are free to move around. You can think of a liquid as one of those ball-pits that one can find at McDonalds.
Gases have the following properties:
1. No fixed volume
2. Conform to shape of container
3. Compressible and expandable
The air you breathe is an example of a gas- a mixture of different gases, to be accurate. Imagine that you bought a new air freshener and put it in the corner of your room. Over time, the molecules from the freshener would expand until the entire room had an equal distribution of the freshener. If you were to open the door of your room, and if the air freshener were unreasonably large, your entire house would smell of it after a period of time. With gases, the molecules will continue to spread/diffuse until they can't diffuse anymore.
Imagine that you had an empty glass. This glass is only empty in the conventional sense: on an atomic scale, the glass is flled with gas molecules (To chemists, the glass is always 100% full).

Now imagine that you attached a piston to the top of the cylinder such that no gas is able to permeate through the piston. If you push down on the piston, does the gas resist this pushing? Of course not! This is because gases are easily compressible. Similarly, if you pull the piston upward as seen in the diagram above, the gas doesn't resist this either seeing as gases will expand to the volume of the container.
When picturing gases, the main distinction between gases and the other states is that gases consist of individual molecules floating around, unbonded to each other.
To conclude, here's a good picture to help visualize the differences between the three states.
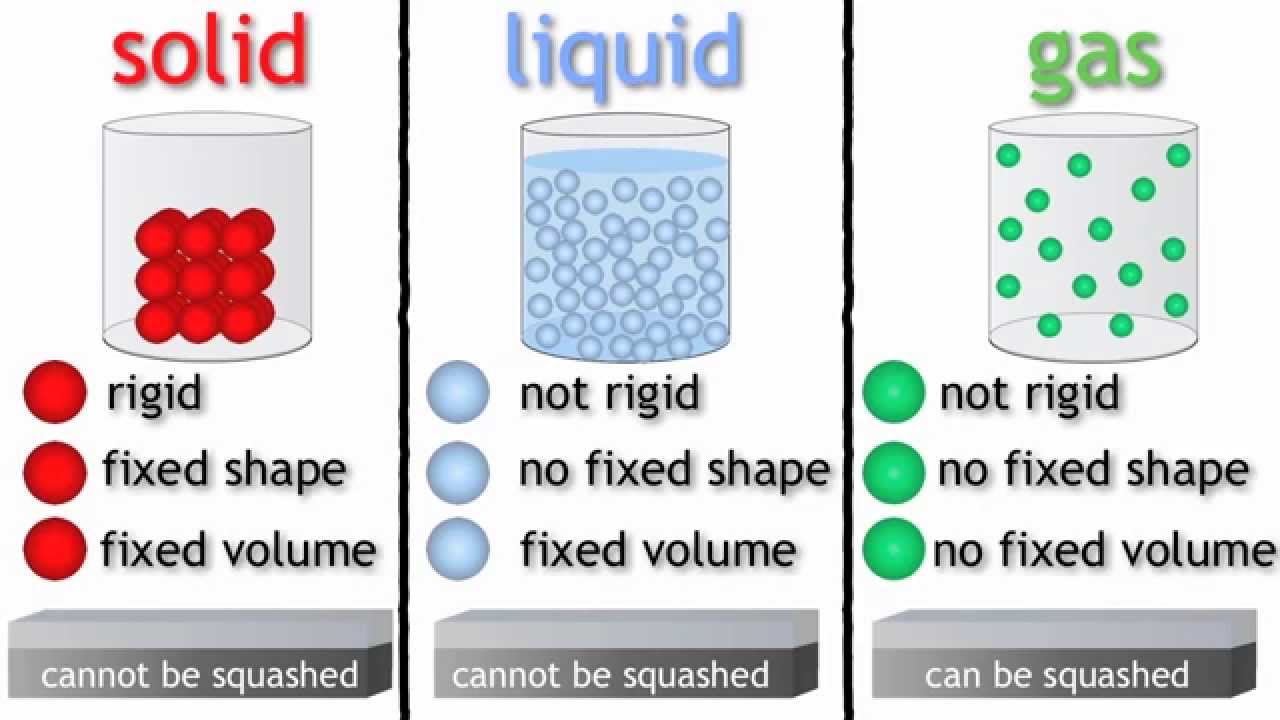
Note: squashed = compressed
1. The state of matter of a material refers to the microscopic physical arrangement of the material.
2. Solids consists of rigidly packed atoms due to the bonding.
3. Liquids consist of some atoms bonded, but not as rigidly as the atoms in a solid.
4. Gases consist of individual molecules floating around, with little to no bonding between the different molecules.
5. Solids and liquids are difficult to compress, whereas gases are easy.
6. Liquids and gases will take on the shape of their container.
1. Gallium
Gallium has a melting point of `30°C` or `85.6°F`, which is just above room temperature but below body temperature. When gallium sits in a room undisturbed, it looks just like any other metal. If you hold it in your hands though, it will melt.
Chemists used to prank guests by making spoons out of gallium and handing them to their guests for their coffee. Once the guests tried to stir the coffee, the spoon would melt, much to the guests' dismay.
2. Pitch Drop Experiment
The line between what constitutes a solid and what constitutes a liquid can be pretty thin for certain materials. With water and ice, it's pretty apparent. With some other materials, its hard to tell whether the material is a really soft solid or a really thick liquid.
The measure of a liquid's "thickness" or resistance to flow is called viscosity. In our everyday lives, we come across this: honey, for example, is a liquid with a viscosity roughly 10,000x that of water. There are currently several viscosity experiments going on throughout the world, called pitch drop experiments, in which extremely viscous liquids are suspended in the air with cameras recording to see when they produce drops. One particularly famous experiment went on Trinity College, Dublin where a total of 7 drops was recorded over the course of a century. The most recent drop happened in 2013 during the few minutes where the person watching the experiment left the room to get coffee. Talk about a missed drop-portunity.
3. Is glass a liquid?
You'll often hear people saying that glass is actually a liquid. This is due to the fact that the glass on old churches, such as the Sistine Chapel, is thicker on the bottom than on top. People who claim that glass is a liquid explain that the glass is thicker on the bottom due to the extremely high viscosity of glass: over time, if glass was a highly viscous liquid, the glass would flow downward due to gravity.
The reality is that glass made at that time often came out uneven due to the creation process. To remedy this, the architects would put the thicker edge of glass on the bottom as opposed to the top. Even with that considered, it's still possible that the glass was developed with initial imbalances and continued to flow over time. If this were the case however, telescope lenses from the time would be unusable now due to the specificity that is required of lenses. As of now, we have yet to find evidence of glass flow. Thus, it is safe to say that glass is an amorphous solid, not a liquid.
4. What are the other states of matter?
There are actually a huge number of exotic states of matter - so many in fact that we don't know all of them. The vast majority of these exotic states are not encountered on a daily basis.
One of the common "exotic" states is plasma, which is present more often than one may think. When lightning strikes, the air that's struck by the lightning is plasma. Plasma is just ionized gas, but behaves vastly differently. Fire is an example of a plasma.
Another state of matter that is significantly more exotic is the Bose-Einstein condensate (BEC). The BEC state appears when a class of particles called bosons are cooled to near absolute 0. When cooled near the necessary temperature, atoms lose their individuality and coalesce into a single macroscopic molecule.