For a brief review of what a titration is, check out the following link:
As a brief summary, titration is a technique used to determine the concentration of an unknown acid or base through the controlled addition of a known concentration of an acid or base.
The setup for a titration involves the unknown acid/base in a beaker and the beaker under a buret as seen below. An indicator, which changes color upon reaching a certain `pH` , is placed in the solution. Alternatively, one can use a `pH` meter to accomplish the same.
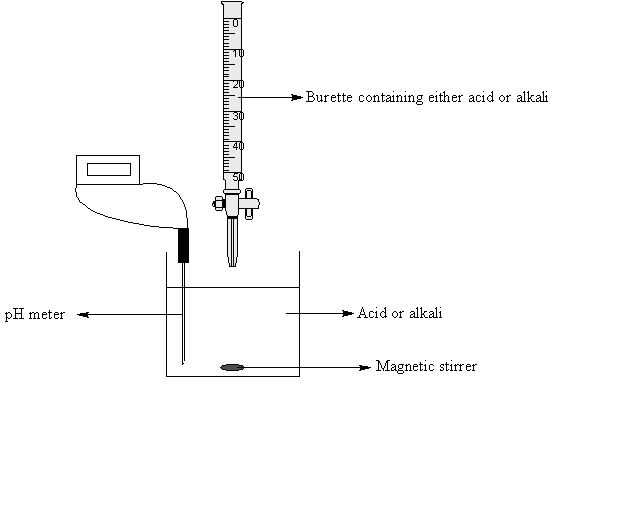
The titrant is then slowly released from the buret until the indicator changes color. At this point, we know the concentration of the titrant, the amount of titrant used, and the amount of the unknown solution. That's all the information needed to calculate the concentration of the unknown solution!
A `pH` curve is a graph that shows the pH of a solution with different amounts of titrants added. For example, look at the following `pH` curve:
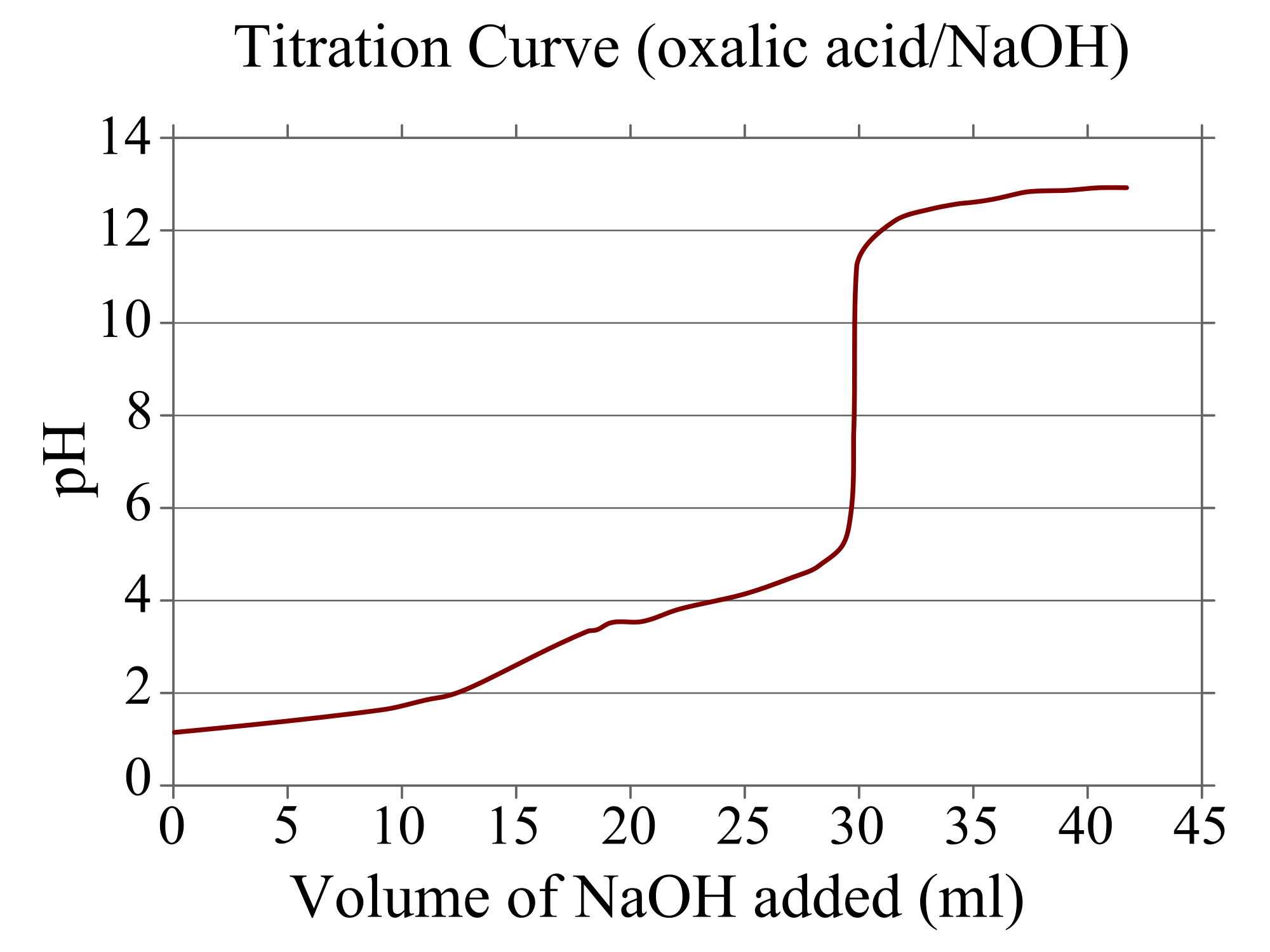
We see that the `pH` of the solution begins at a little over 1, but increases as the amount of NaOH is added.
The equivalence point of the curve is the point in equal amounts of acid and base have been mixed. In other words, the equivalence point is where the concentrations of `[H^+]` and `[OH^-`] are the same. The end point is the point in which the indicator changes color. One can think of the equivalence point as the point in which the titration should end, whereas the end point is the point in which the titration actually ends in practice.
The equivalence and end points can be the same for a given titration, but are usually not. The reason for this is that most indicators change color at a different `pH` than `pH=7` e.g phenolphthalein will change into a deep pink color at `pH 10-13` , but is colorless at all other `pH` . For an acid-base titration where phenolphthalein is the indicator, the equivalence point is still the point at which `[H^+]=[OH^-]` , but the end point is where the `pH=10` .
To summarize:
1. The equivalence point is the point in which the `[H^+]=[OH^-]` .
2. The end point is the point in which the indicator changes color.
3. The equivalence point and end point can be, but are usually not, the same.
In a strong acid-strong base titration, a strong acid is used to titrate a strong base, or vice versa. For these titrations, we can assume that the species dissociate fully. In other words, we have:
`[H^+]=["Strong Acid"]` and `[OH^-]=["Strong Base"]`