Let's say that you're the manager of a car factory and need to order new parts to build more cars. In order to build 1 car, you need 1 frame, 4 tires, and 2 side mirrors (Let's assume you're building a futuristic car that doesn't require an engine or a steering wheel). You want to build 100 cars, so you do the math and order 100 bodies, 400 tires, and 200 side mirrors. Once the order arrives, you disappointingly discover that the delivery company broke 10 of the mirrors in transit. How does this affect your production?
Obviously you can no longer build all 100 cars. Without the 10 mirrors, 5 of the cars cannot be built. The mirrors are going to limit how many cars you can build. Even after all the mirrors have been used, there will be remaining tires and bodies that cannot be used.
The same principle applies to chemistry. In real life, reactions usually do not have the perfect amounts of reactants needed in order to fully react. The limiting reagent or limiting reactant is the reactant that there is a lack of. If this is confusing, think of it this way: the limiting reactant is the reactant that runs out first. Consider the reaction:
`4Al + 3O_2 rArr 2Al_2O_3`.
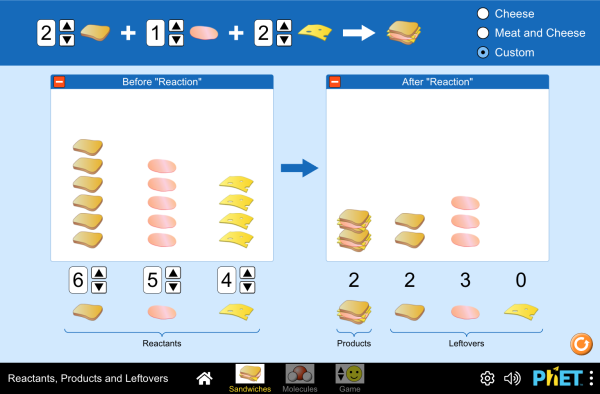
For every mole of `Al` you have, you need `3/4 "mol" O_2` to react with it. If you don't have this amount, what happens? Here's a simulation to help illuminate the concept. I recommend playing around with the sandwiches first, then moving onto actual molecules.
#1. You have 107.5 g Al and 64 g `O_2`.
a) Calculate the amount of `Al_2O_3` formed in the reaction `4Al + 3O_2 rArr 2Al_2O_3`
First step as always is to convert to moles.
`(107.5 g Al)((1 mol)/(26.982 g Al))=4 "mol" Al`
`(64 g O_2)((1 "mol")/(32 g O_2))=2 "mol" O_2`
Note: The molecular weight of `O_2` is `(32 g)/("mol")` because there are 2 oxygen atoms per molecule of `O_2.`
You can probably tell where this is headed. The equation calls for `4 "mol" Al` and `3 "mol" O_2`, but we only have `2 "mol" O_2`. Just from that, we know that `O_2` is going to be our limiting reactant. Since the `O_2` runs out first, the `O_2` now determines how much of the product is formed. It will usually not be this easy to identify the limiting reactant, as you'll see in practice problems soon.
Now we just have to convert the amount of `O_2` we have into `Al_2O_3`. We don't have to worry about the `Al` because that's in excess compared to the `O_2`; even after we use up all of our `O_2`, some `Al` will remain unreacted.
`(2 "mol" O_2)((2 "mol" Al_2O_3)/(3 "mol" O_2))=4/3 "mol" Al_2O_3`
`(4/3 "mol" Al_2O_3)((102 g Al_2O_3)/(1 "mol" Al_2O_3))=136 g Al_2O_3`
Answer: In total, `136 g` of `Al_2O_3` will be produced.
If the conversion factors don't make sense, consider reviewing the previous sections:
b) Which one of the reactants is the limiting reactant?
The limiting reactant is the reactant that runs out first. In this reaction, `O_2` runs out first. Therefore, `O_2` is the limiting reactant.
c) Which one of the reactants is in excess? How much of this reactant remains?
If `O_2` is the limiting reactant, `Al` must be in excess. To calculate how much `Al` remains, we simply have to look at how much was used in the reaction with `O_2`.
`(2 "mol" O_2)((4 "mol" Al)/(3 "mol" O_2))=8/3 "mol" Al`
`(8/3 "mol" Al)((26.982 g Al)/(1 mo))=71.7 g Al`
This means that `71.7 g` of `Al` was used to react with the entirety of `O_2.` This should be less `Al` than we have in total since not all of it reacted. We started off with `4 "mol" Al` ; let's see how much is left.
`(4 "mol" Al)((26.892 g Al)/(1 "mol"))=107.6 g Al`
This means we used less `Al` than we started off with! This makes sense since the `Al` is in excess. To see how much `Al` we have left, we simply have to subtract the amount we used from the initial amount.
`Initial_(Al)-Reacted_(Al)=107.6 g-71.7 g=29.9 g`
Answer: `29.9 g`
In conclusion, we have `29.9 g` `Al` left over after all of the `O_2` runs out. Let's sum what we've done in this problem:
1. Converted all given amounts to moles.
2. Determined the limiting reactant by looking at the chemical equation.
3. Convert the moles of limiting reactant to moles of product by using the conversion factor.
4. Convert the moles product to grams product.
5. Determined the amount of Al in excess by converting moles of the limiting reactant to grams Al.
I highly recommend you go through the problem and make sure you understand why each step was taken and the mathematical process behind each step. Instead of memorizing the steps, make sure you understand the purpose behind each one. In the final review of this section, we'll go over more difficult limiting reagent problems.